Synopsis
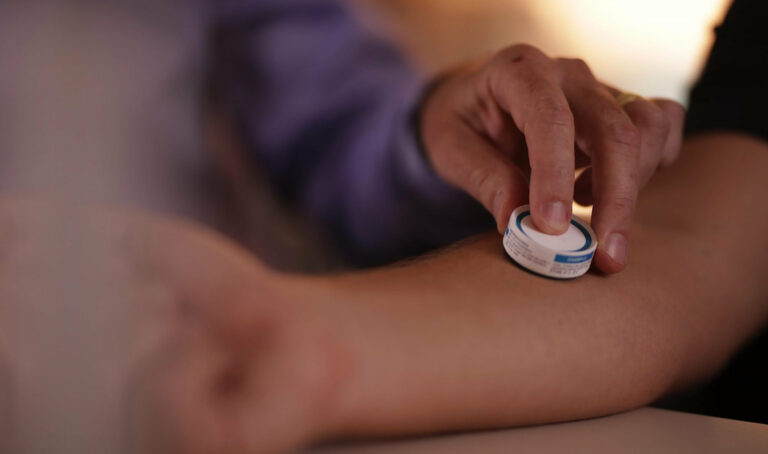
Vaxxas is a biotech company that is developing a new way to deliver vaccines using high-density microarray patch (HD-MAP) that is applied to the skin for a few seconds instead of being injected via a needle. This technology has the potential to greatly improve the efficiency and accessibility of vaccinations around the world.
HD-MAP utilizes a micro-needling technology that painlessly delivers the vaccine through the skin, which has the potential to be self-administered, reducing the need for trained medical professionals to administer the vaccine. The HD-MAP also eliminates the risk of needle-stick injuries and the need for cold storage, making the vaccine more stable and easier to transport.
This technology could greatly improve the speed and efficiency of vaccination campaigns, which would have a tremendous impact on public health by helping to prevent the spread of deadly diseases. It could also help to increase vaccination rates, especially in hard-to-reach populations, and could ultimately save hundreds of thousands of lives each year.
testimonials

David Hoey
CEO, Vaxxas
OneVentures have been terrific to work with as a lead investor, but it’s what they add in addition to investment that has been critical to Vaxxas’ growth.
Their biotech team comes from leadership roles with hands-on industry experience spanning technology to business development. Vaxxas is tremendously fortunate – working with the OneVentures team has translated into sharpening all aspects of our development and commercialisation strategy, and actively helped us forge connections and partners with industry. I cannot recommend them highly enough.












